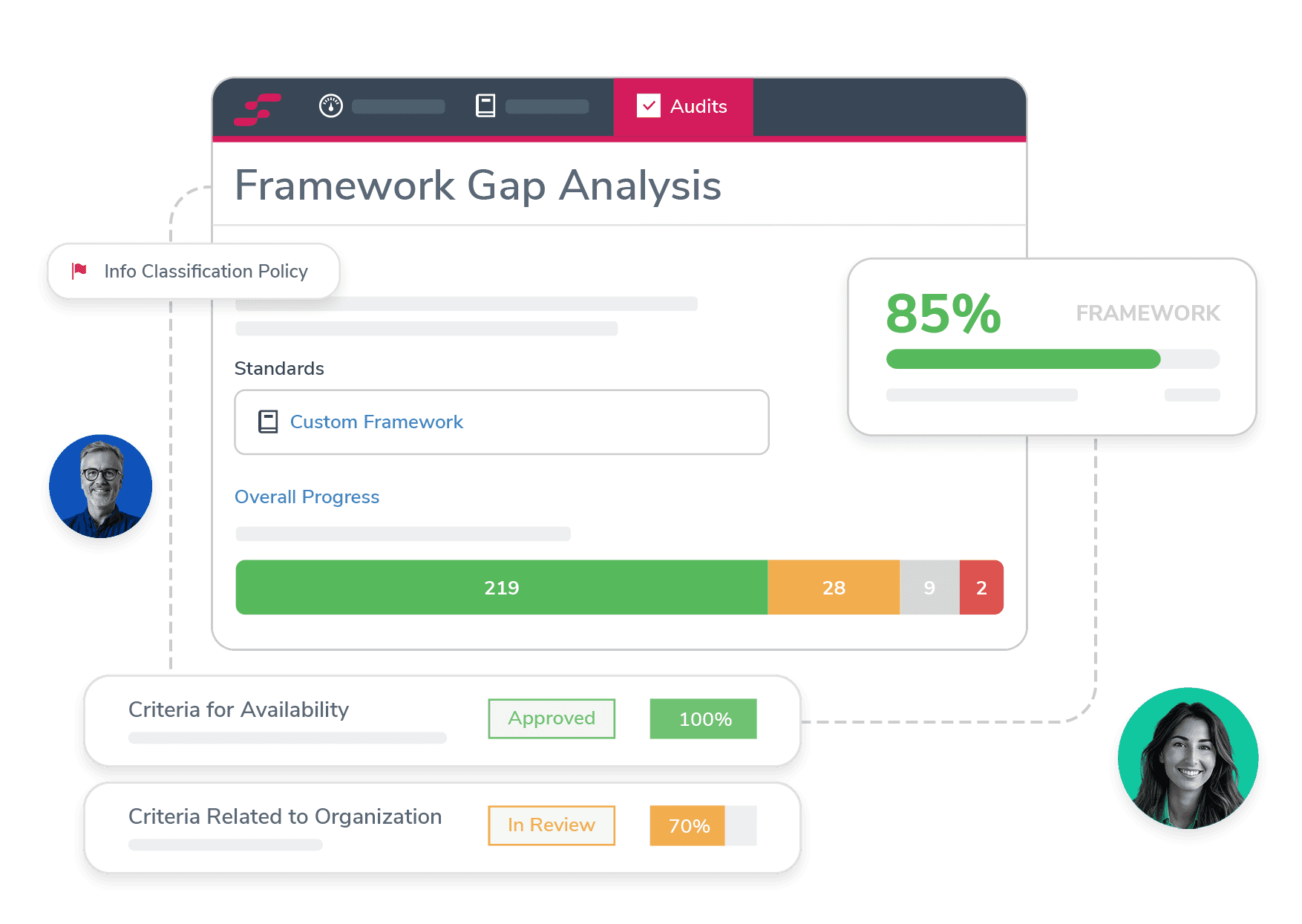
Easily Manage ICH GCP
StandardFusion helps ensure ICH GCP compliance by centralizing the management of clinical protocols and automating the tracking of regulatory requirements, making it easier for organizations to adhere to global standards and maintain data integrity.
International Council for Harmonisation Good Clinical Practice
Description
The ICH GCP (International Council for Harmonisation Good Clinical Practice) guidelines are an international ethical and scientific quality standard for designing, conducting, recording, and reporting clinical trials that involve human subjects. These guidelines aim to protect the rights, safety, and well-being of trial participants while ensuring that clinical trial data is credible and accurate. ICH GCP is widely recognized and used globally to ensure that clinical trials are conducted in a consistent and reliable manner, facilitating the acceptance of clinical trial data across different regions.
Overview
The ICH GCP guidelines are intended for organizations involved in clinical research, including pharmaceutical companies, clinical research organizations, and academic institutions. The purpose is to ensure that clinical trials are conducted ethically and with high scientific standards, protecting participants and ensuring reliable data for regulatory submissions.